Immobilization of Dibenzalacetone on TiO2 Surface and its Potential as Anti-UV Material
DOI:
https://doi.org/10.22437/chp.v7i1.26109Keywords:
dibenzalacetone, TiO2, immobilization, anti-UVAbstract
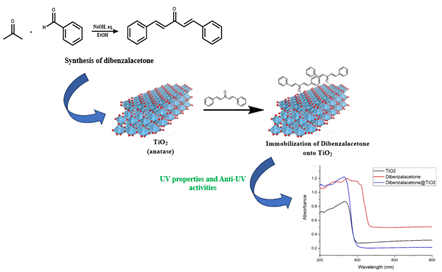
Sunlight has been known to provide many benefits to human life. However, behind these benefits, there are some negative effects along with the destruction of the ozone layer and the environment on earth. One of them is exposure to ultraviolet (UV) rays which can cause several diseases such as skin cancer. One way to overcome this is by using sunscreen substances. In this study, dibenzalacetone immobilization on TiO2 has been carried out for anti-UV applications. Based on the test results using UV-Vis spectrometry, TiO2, and dibenzalacetone both have anti-UV properties with maximum peaks at wavelengths of 335 nm and 346 nm with absorbance values of 0.871 and 1.197. Immobilization of TiO2 with dibenzalacetone gives an absorbance with a value of 1.221 at a wavelength of 329. These results indicate that TiO2 immobilization with dibenzalacetone provides better anti-UV A properties than TiO2 because of the higher absorbance value.
Downloads
References
Wang, X., Sun, Q., Gao, J., Wang, J., Xu, C., Ma, X., & Zhang, F. (2021). Recent Progress of Organic Photovoltaics with Efficiency over 17%. Energies, 14(14), 4200. https://doi.org/10.3390/en14144200
Stone, K. A., Solomon, S., Kinnison, D. E., & Mills, M. J. (2021). On Recent Large Antarctic Ozone Holes and Ozone Recovery Metrics. Geophysical Research Letters, 48(22). https://doi.org/10.1029/2021GL095232
Kollias, N., & Baqer, A. (1984). An experimental study of the changes in pigmentation in human skin in vivo with visible and near infrared light. Photochemistry and Photobiology, 39(5), 651–659. https://doi.org/10.1111/j.1751-1097.1984.tb03905.x
Mahmoud, B. H., Ruvolo, E., Hexsel, C. L., Liu, Y., Owen, M. R., Kollias, N., Lim, H. W., & Hamzavi, I. H. (2010). Impact of Long-Wavelength UVA and Visible Light on Melanocompetent Skin. Journal of Investigative Dermatology, 130(8), 2092–2097. https://doi.org/10.1038/jid.2010.95
Pfeifer, G. P. (2020). Mechanisms of UV-induced mutations and skin cancer. Genome Instability & Disease, 1(3), 99–113. https://doi.org/10.1007/s42764-020-00009-8
Appannagari, R. R. (2017). Environmental pollution causes and consequences: a study. North Asian International Research Journal of Social Science & Humanities, 3(8), 151–161.
Rahmawati, R., Muflihunna, A., & Amalia, M. (2018). Analisis aktivitas perlindungan sinar uv sari buah sirsak (annona muricata l.) berdasarkan nilai Sun Protection Factor (SPF) secara spektrofotometri UV-VIS. Jurnal Fitofarmaka Indonesia, 5(2), 284–288. https://doi.org/10.33096/jffi.v5i2.412
Leiter, U., Eigentler, T., & Garbe, C. (2014). Epidemiology of Skin Cancer. In Sunlight, Vitamin D and Skin Cancer (pp. 120–140). Springer New York. https://doi.org/10.1007/978-1-4939-0437-2_7
Carr, S., Smith, C., & Wernberg, J. (2020). Epidemiology and Risk Factors of Melanoma. Surgical Clinics of North America, 100(1), 1–12. https://doi.org/10.1016/j.suc.2019.09.005
Markovitsi, D. (2016). UV-induced DNA Damage: The Role of Electronic Excited States. Photochemistry and Photobiology, 92(1), 45–51. https://doi.org/10.1111/php.12533
Putri, Y. D., Kartamihardja, H., & Lisna, I. (2019). Formulasi dan Evaluasi Losion Tabir Surya Ekstrak Daun Stevia (Stevia rebaudiana Bertoni M). Jurnal Sains Farmasi & Klinis, 6(1), 32. https://doi.org/10.25077/jsfk.6.1.32-36.2019
Catalano, R., Labille, J., Gaglio, D., Alijagic, A., Napodano, E., Slomberg, D., Campos, A., & Pinsino, A. (2020). Safety Evaluation of TiO2 Nanoparticle-Based Sunscreen UV Filters on the Development and the Immunological State of the Sea Urchin Paracentrotus lividus. Nanomaterials, 10(11), 2102. https://doi.org/10.3390/nano10112102
Sharma, S., Sharma, R. K., Gaur, K., Cátala Torres, J. F., Loza-Rosas, S. A., Torres, A., Saxena, M., Julin, M., & Tinoco, A. D. (2019). Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials, 12(14), 2317. https://doi.org/10.3390/ma12142317
Borges, I. D., Danielli, J. A. V., Silva, V. E. G., Sallum, L. O., Queiroz, J. E., Dias, L. D., Iermak, I., Aquino, G. L. B., Camargo, A. J., Valverde, C., Osório, F. A. P., Baseia, B., & Napolitano, H. B. (2020). Synthesis and structural studies on (E)-3-(2,6-difluorophenyl)-1-(4-fluorophenyl)prop-2-en-1-one: a promising nonlinear optical material. RSC Advances, 10(38), 22542–22555. https://doi.org/10.1039/D0RA03634J
Bargujar, S., & Ratnani, S. (2022). An Alternative Greener Synthesis of Dibenzalacetone. Organic Preparations and Procedures International, 54(6), 563–565. https://doi.org/10.1080/00304948.2022.2096383
de Oliveira, M. M., Nogueira, C. E. S., Almeida-Neto, F. W. Q., Santos, H. S., Teixeira, A. M. R., de Lima-Neto, P., Marinho, E. S., de Moraes, M. O., Pessoa, C., & Barros-Nepomuceno, F. W. A. (2021). Full Spectroscopic Characterization and Cytotoxicity Activity of Synthetic Dibenzalacetone Derivatives. Journal of Molecular Structure, 1231, 129670. https://doi.org/10.1016/j.molstruc.2020.129670
Budiati, T., Soewandi, A., & Soegianto, L. (2019). Microwave-assisted Synthesis of Dibenzalacetone Derivatives and Study of their Potential Antioxidant Activities. Journal of Chemical and Pharmaceutical Research, 11(9), 11–16.
Handayani, S., Matsjeh, S., Anwar, C., Atun, S., & Fatimah, I. (2012). Novel Synthesis of 1,5-dibenzalacetone Using NaOH/ZrO2- Montmorillonite as Cooperative Catalyst. International Journal of Chemical and Analytical Science, 3(3), 1419–1424.
Sudha, S., Sundaraganesan, N., Vanchinathan, K., Muthu, K., & Meenakshisundaram, SP. (2012). Spectroscopic (FTIR, FT-Raman, NMR and UV) and molecular structure investigations of 1,5-diphenylpenta-1,4-dien-3-one: A combined experimental and theoretical study. Journal of Molecular Structure, 1030, 191–203. https://doi.org/10.1016/j.molstruc.2012.04.030
Dharma, J., Pisal, A., & Shelton, C. T. (2009). Simple method of measuring the band gap energy value of TiO2 in the powder form using a UV/Vis/NIR spectrometer. Application Note Shelton, CT: PerkinElmer, 1–4.