Anticancer Activities of Seven Peronemins (A2, A3, B1, B2, B3, C1, and D1) from Peronema canescens Jack: A Prediction Studies
DOI:
https://doi.org/10.22437/chp.v7i1.23726Kata Kunci:
anticancer, peronemins, Peronema canescens JackAbstrak
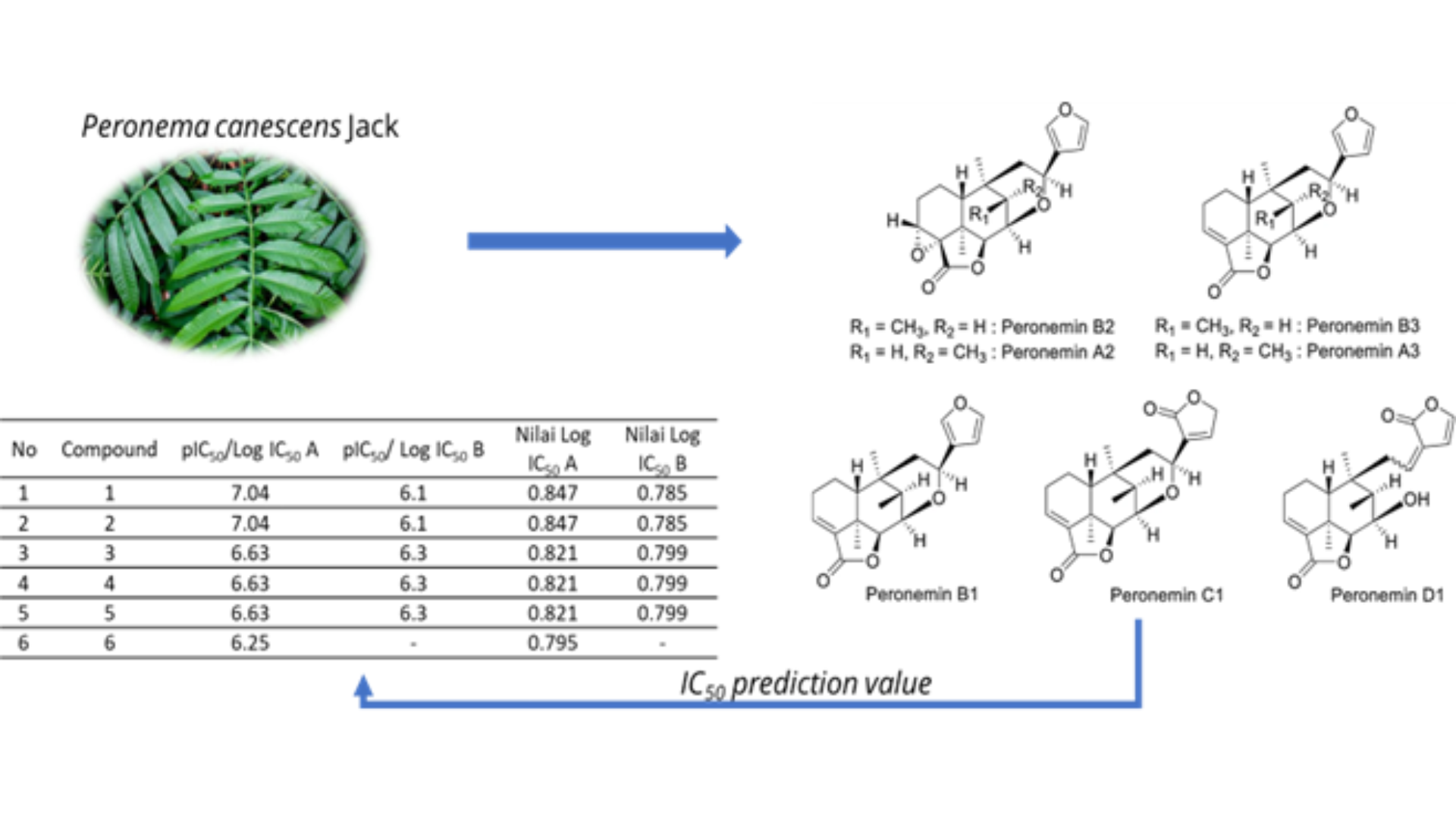
Cancer is one of the leading causes of human death. In 2019, it was reported that cancer was the second (22%) cause of death due to non-communicable diseases in the world's population. Research for alternative anticancer drugs is still being done, including anticancer from plants. One of the plants that have the potential to be developed as an anticancer alternative is the sungkai plant. Sungkai leaves contain many bioactive compounds, one of which is the clerodane-type diterpenoids, peronemins, A2 (1), A3 (2), B1 (3), B2 (4), B3 (5), C1 (6), and D1 (7). The aim of this study was to initial screen the potential of seven Peronemins compounds in Sungkai leaves extract as anticancer candidates. Initial screening was carried out by predicting in-silico anticancer activity of the seven compounds. Dihydrofolate reductase inhibitor (DHFR inhibitor) is one of the anticancer activity screening approaches. DHFR Inhibitor activity from perenomins derivatives with pIC50 values ​​of 0.785 (A2), respectively; 0.785 (A3); 0.799 (B2); 0.799 (B3); 0.799 (C1 and D1). In addition, from compounds 1,2,3,4,5 peronemin derivatives have potential anticancer activity through interaction with the target protein Voltage-gated potassium channel subunit while compounds 6, 7 also have biological activity potential anticancer on target protein Dihydrofolate reductase.
Unduhan
Referensi
Andania, M. M., Ismed, F., Taher, M., Ichwan, S. J. A., Bakhtiar, A., & Arbain, D. (2019). Cytotoxic Activities of Extracts and Isolated Compounds of Some Potential Sumatran Medicinal Plants against MCF-7 and HSC-3 Cell Lines. Journal of Mathematical and Fundamental Sciences, 51(3), 225–242. https://doi.org/10.5614/j.math.fund.sci.2019.51.3.2
Amandito, R., & Viryawan, C. (2013). The Characteristics of Breast Cancer Patients in Dharmais Hospital National Cancer Center Jakarta Based on Occupational and Environmental Status. Indonesian Journal of Cancer, 7(2), 53.
Ng, C. H., Pathy, N. B., Taib, N. A., Teh, Y. C., Mun, K. S., Amiruddin, A., Evlina, S., Rhodes, A., & Yip, C. H. (2011). Comparison of breast cancer in Indonesia and Malaysia--a clinico-pathological study between Dharmais Cancer Centre Jakarta and University Malaya Medical Centre, Kuala Lumpur. Asian Pacific Journal of Cancer Prevention : APJCP, 12(11), 2943–2946.
Warnakulasuriya, S. (2009). Global epidemiology of oral and oropharyngeal cancer. Oral Oncology, 45(4–5), 309–316. https://doi.org/10.1016/j.oraloncology.2008.06.002
Latief, M., Fisesa, A. T., Sari, P. M., & Tarigan, I. L. (2021). Anti Inflammatory Activity of Sungkai Leaves (Peronema canescens Jack) Ethanol Extract In Carrageenan Induced Mice. Jurnal Farmasi Sains Dan Praktis, 7(2), 144–153. https://doi.org/10.31603/pharmacy.v7i2.4532
Gutman, G. A., Chandy, K. G., Grissmer, S., Lazdunski, M., Mckinnon, D., Pardo, L. A., Robertson, G. A., Rudy, B., Sanguinetti, M. C., Stühmer, W., & Wang, X. (2005). International Union of Pharmacology. LIII. Nomenclature and Molecular Relationships of Voltage-Gated Potassium Channels. Pharmacological Reviews, 57(4), 473–508. https://doi.org/10.1124/pr.57.4.10
Szabò, I., Bock, J., Jekle, A., Soddemann, M., Adams, C., Lang, F., Zoratti, M., & Gulbins, E. (2005). A Novel Potassium Channel in Lymphocyte Mitochondria. Journal of Biological Chemistry, 280(13), 12790–12798. https://doi.org/10.1074/jbc.M413548200
Jang, S. H., Byun, J. K., Jeon, W.-I., Choi, S. Y., Park, J., Lee, B. H., Yang, J. E., Park, J. B., O’Grady, S. M., Kim, D.-Y., Ryu, P. D., Joo, S.-W., & Lee, S. Y. (2015). Nuclear Localization and Functional Characteristics of Voltage-gated Potassium Channel Kv1.3. Journal of Biological Chemistry, 290(20), 12547–12557. https://doi.org/10.1074/jbc.M114.561324
Zhu, J., Yan, J., & Thornhill, W. B. (2014). The Kv1.3 potassium channel is localized to the cis-Golgi and Kv1.6 is localized to the endoplasmic reticulum in rat astrocytes. FEBS Journal, 281(15), 3433–3445. https://doi.org/10.1111/febs.12871
Panyi, G., Possani, L., de la Vega, R. C., Gaspar, R., & Varga, Z. (2006). K+ Channel Blockers: Novel Tools to Inhibit T Cell Activation Leading to Specific Immunosuppression. Current Pharmaceutical Design, 12(18), 2199–2220. https://doi.org/10.2174/138161206777585120
Wulff, H., Castle, N. A., & Pardo, L. A. (2009). Voltage-gated potassium channels as therapeutic targets. Nature Reviews Drug Discovery, 8(12), 982–1001. https://doi.org/10.1038/nrd2983
Cahalan, M. D., & Chandy, K. G. (2009). The functional network of ion channels in T lymphocytes. Immunological Reviews, 231(1), 59–87. https://doi.org/10.1111/j.1600-065X.2009.00816.x
Feske, S., Wulff, H., & Skolnik, E. Y. (2015). Ion Channels in Innate and Adaptive Immunity. Annual Review of Immunology, 33(1), 291–353. https://doi.org/10.1146/annurev-immunol-032414-112212
Teisseyre, A., Palko-Labuz, A., Sroda-Pomianek, K., & Michalak, K. (2019). Voltage-Gated Potassium Channel Kv1.3 as a Target in Therapy of Cancer. Frontiers in Oncology, 9. https://doi.org/10.3389/fonc.2019.00933
Srinivasan*, B., Tonddastâ€Navaei, S., Roy, A., Zhou, H., & Skolnick, J. (2019). Chemical space of Escherichia coli dihydrofolate reductase inhibitors: New approaches for discovering novel drugs for old bugs. Medicinal Research Reviews, 39(2), 684–705. https://doi.org/10.1002/med.21538