Molecular Docking Study of Chalcone Analogue Compounds with Hydroxy and Methoxy Subtituents as Bcl-2 Inhibitors
DOI:
https://doi.org/10.22437/chp.v7i1.17487Keywords:
chalcone analogue compound, molecular docking, senyawa analog kalkon, molecular docking, venetoclax, inhibitor Bcl-2, Bcl-2 inhibitorsAbstract
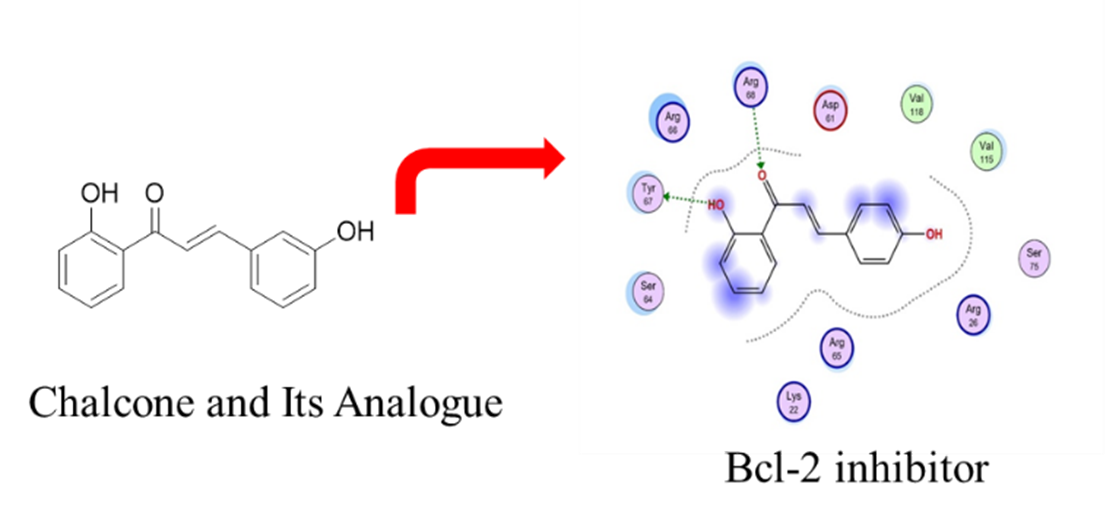
Molecular docking study of 6 chalcone analogues with protein target from the crystallographic structure modeling of Bcl-2 protein with PDB code 2W3L was carried out using computational media using the Molecular Operating Environment (MOE) program. The aim of this study is to determine the potentiality of the 6 chalcone analogue compounds as Bcl-2 inhibitors using molecular docking studies. In this study, venetoclax was used as positive control. Based on docking results, binding free energy was used as information to know which wheather chalcone analogue compounds are active or not as Bcl-2 inhibitors. According to the docking results that have been carried out, it showed that the 6 chalcone analogue compounds have no potential as Bcl-2 inhibitors. Due to the superimposition of the 6 compounds that did not stick to the positive control and most importantly the binding free energy values ​​(S) of the 6 chalcone analogue compounds were higher than the binding free energy values ​​of the positive control (Venetoclax).
Downloads
References
Indrawati, M. (2009). Bahaya kanker bagi wanita dan pria : Pengenalan, penanganan dan pencegahan terhadap kanker : Diantaranya kanker serviks, kanker payudara, kanker rahim, kanker prostat, kanker hati, kanker kulit, dan lain-lain. AV.
Kumar, V., Abbas, A., & Aster, J. C. (2017). Robbins Basic Pathology (10 ed). Elsevier Health Sciences.
International Agency for Research on Cancer, & W. H. O. (2018). Incidence, Mortality and Prevalence by Cancer Site. Globocan.
Mangan, Y. (2009). Solusi Sehat Mencegah & Mengatasi Kanker. AgroMedia Pustaka.
Djajanegara, I., & Wahyudi, P. (2010). Uji sitotoksisitas ekstrak etanol herba Ceplukan (Physalis angulata Linn.) terhadap sel T47D secara in vitro. Urnal Ilmu Kefarmasian Indonesia, 8(1), 41–47.
Pratoko, D. K. (2012). Molecular Docking Turunan Kalkon Terhadap Reseptor Estrogen B (ER-B) Sebagai Antikanker Payudara. Jurnal Kimia Terapan Indonesia, 14(1), 20–25.
Mahapatra, D. K., Bharti, S. K., & Asati, V. (2015). Anti-cancer chalcones: Structural and molecular target perspectives. European Journal of Medicinal Chemistry, 98, 69–114. https://doi.org/10.1016/j.ejmech.2015.05.004
Oktaviani, R., Arifian, H., Rahmadani, A., Zamruddin, N. M., & Rusli, R. (2019). Kajian In Silico Senyawa Turunan Kalkon sebagai Antikanker. Proceeding of Mulawarman Pharmaceuticals Conferences, 9, 22–26. https://doi.org/10.25026/mpc.v9i1.309
Mohamed, M. F., Mohamed, M. S., Shouman, S. A., Fathi, M. M., & Abdelhamid, I. A. (2012). Synthesis and Biological Evaluation of a Novel Series of Chalcones Incorporated Pyrazole Moiety as Anticancer and Antimicrobial Agents. Applied Biochemistry and Biotechnology, 168(5), 1153–1162. https://doi.org/10.1007/s12010-012-9848-8
Dona, R., Zamri, A., & Jasril, -. (2015). Sintesis Dan Uji Toksisitas Senyawa Analog Kalkon Tersubstitusi Metoksi. Photon: Jurnal Sain Dan Kesehatan, 5(2), 9–14. https://doi.org/10.37859/jp.v5i2.579
Suwito, H., Jumina, Mustofa, Ni’matuzahroh, & Puspaningsih, N. N. T. (2015). Anticancer and antimicrobial activity of methoxy amino chalcone derivatives. Der Pharma Chemica, 7(3), 89–94.
Anwar, C., Prasetyo, Y. D., Matsjeh, S., Haryadi, W., Sholikhah, E. N., & Nendrowati, N. (2018). Synthesis of Chalcone Derivatives and Their <i>in vitro</i> Anticancer Test Against Breast (T47D) and Colon (WiDr) Cancer Cell Line. Indonesian Journal of Chemistry, 18(1), 102. https://doi.org/10.22146/ijc.26864
Phillips, M. A., Stewart, M. A., Woodling, D. L., & Xie, Z.-R. (2018). Has Molecular Docking Ever Brought us a Medicine? In Molecular Docking. InTech. https://doi.org/10.5772/intechopen.72898
Frimayanti, N., Mora, E., & Anugrah, R. (2018). Study of Molecular Docking of Chalcone Analoque Compound as Inhibitors for Liver Cancer Cells HepG2. Computer Engineering and Applications, 7(2), 137–147. https://doi.org/https://doi.org/10.18495/comengapp.v7i2.260
Frimayanti, N., Yaeghoobi, M., Ikhtiarudin, I., Putri, D. R. W., Namavar, H., & Bitaraf, F. S. (2020). Insight on the In Silico Study and Biological Activity Assay of Chalcone-Based 1, 5-Benzothiazepines as Potential Inhibitor for Breast Cancer MCF7. Chiang Mai University Journal of Natural Sciences, 20(1), 1–11. https://doi.org/10.12982/CMUJNS.2021.019
Mokhtari, M. J., Akbarzadeh, A., Hashemi, M., Javadi, G., Mahdian, R., Mehrabi, M. R., Farhangi, A., & Mohammadi, H. (2012). Cisplatin Induces Down Regulation of BCL2 in T47D Breast Cancer Cell Line. Advanced Studies in Biology, 4(1), 19–25.
Lok, S. W., Whittle, J. R., Vaillant, F., Teh, C. E., Lo, L. L., Policheni, A. N., Bergin, A. R. T., Desai, J., Ftouni, S., Gandolfo, L. C., Liew, D., Liu, H. K., Mann, G. B., Moodie, K., Murugasu, A., Pal, B., Roberts, A. W., Rosenthal, M. A., Shackleton, K., … Lindeman, G. J. (2019). A Phase Ib Dose-Escalation and Expansion Study of the BCL2 Inhibitor Venetoclax Combined with Tamoxifen in ER and BCL2–Positive Metastatic Breast Cancer. Cancer Discovery, 9(3), 354–369. https://doi.org/10.1158/2159-8290.CD-18-1151
Yaeghoobi, M. (2012). Synthesis of chalcone-based six and seven membered heterocyclic compounds and their biological activities against H1N1 virus. University of Malaya.
Prieto-MartÃnez, F. D., Arciniega, M., & Medina-Franco, J. L. (2018). Acoplamiento Molecular: Avances Recientes y Retos. TIP Revista Especializada En Ciencias QuÃmico-Biológicas, 21(1), 65–87. https://doi.org/10.22201/fesz.23958723e.2018.0.143
Monika, G., Punam, G., Sarbjot, S., & D, G. G. (2010). An overview on Molecular Docking. International Journal of Drug Development & Research, 2(2), 219–231.
Hamzah, N., Haeria, & Paewa, K. D. (2015). Studi Farmakofor dan Docking Molekul Reseptor Σ2 Sebagai Target Pengobatan Kanker Payudara. Jurnal Farmasi UIN Alauddin Makassar, 3(1), 11–16.
Schaefer, T., Wildman, T. A., Sebastian, R., & McKinnon, D. M. (1984). The phenyl group as a hydrogen bond acceptor in 2-phenylphenol derivatives. Substituent comparisons. Canadian Journal of Chemistry, 62(12), 2692–2696. https://doi.org/10.1139/v84-457
Bose, P., Gandhi, V., & Konopleva, M. (2017). Pathways and mechanisms of venetoclax resistance. Leukemia & Lymphoma, 58(9), 2026–2039. https://doi.org/10.1080/10428194.2017.1283032
Pradani, T. C., Fatimawali, ., Manampiring, A. E., Kepel, B. J., Budiarso, F. D., & Bodhi, W. (2021). Molecular Docking Terhadap Senyawa Kurkumin dan Arturmeron pada Tumbuhan Kunyit (Curcuma Longa Linn.) yang Berpotensi Menghambat Virus Corona. Jurnal E-Biomedik, 9(2), 208–214. https://doi.org/10.35790/ebm.v9i2.31888
Xu, Z., Wang, X., Chen, X., Zeng, S., Qian, L., Wei, J., Gong, Z., & Yan, Y. (2019). Identification of Aloperine as an anti-apoptotic Bcl2 protein inhibitor in glioma cells. PeerJ, 7, e7652. https://doi.org/10.7717/peerj.7652
Frimayanti, N., & Zamri, A. (2021). Desain Obat Baru dengan Pendekatan Komputasi. Farha Pustaka.